
Coarse-Graining Conformational Dynamics with Multidimensional Generalized Langevin Equation: How, When, and Why

Enhanced Deep Potential Model for Fast and Accurate Molecular Dynamics: Application to the Hydrated Electron
Ruiqi Gao, Yifan Li, Roberto Car, Phys. Chem. Chem. Phys., 2024,26, 23080 (2024)
URL: https://doi.org/10.1039/D4CP01483A
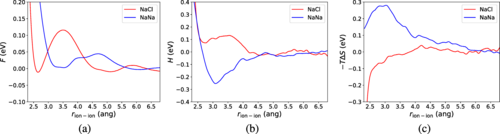
Anti-Coulomb ion-ion interactions: A theoretical and computational study
Alec Wills, Anthony Mannino, Isidro Losada, Sara G. Mayo, Jose M. Soler, Marivi Fernandez-Serra, Physical Review Research, 6, 033095, (2024)
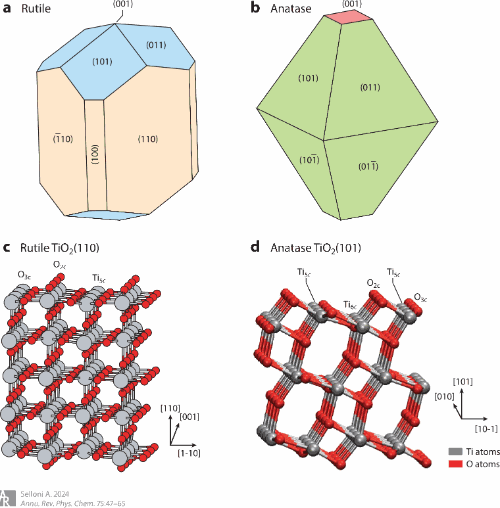
Aqueous titania interfaces
Selloni, A., Annual Review of Physical Chemistry, 2024, 75, (invited review article)
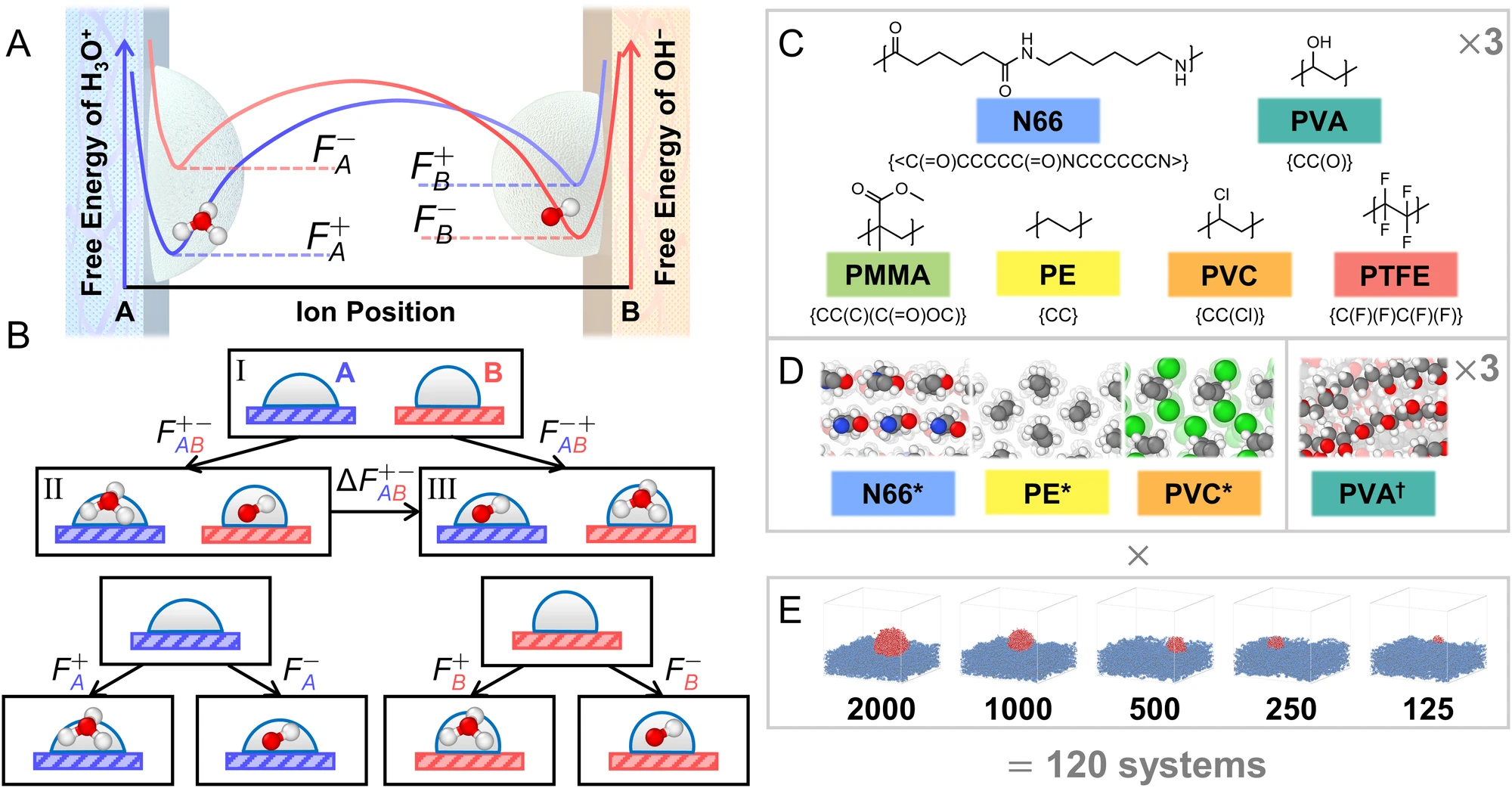
Thermodynamic driving forces in contact electrification between polymeric materials
Zhang, H., Sundaresan, S. & Webb, M.A., Nat Commun 15, 2616 (2024)
Understanding Strain and Failure of a Knot in Polyethylene using Molecular Dynamics with Machine-Learned Potentials
Mark DelloStritto and Michael Klein, J. Phys. Chem. Lett. 2024, 15, 35, 9070–9077 (2024)
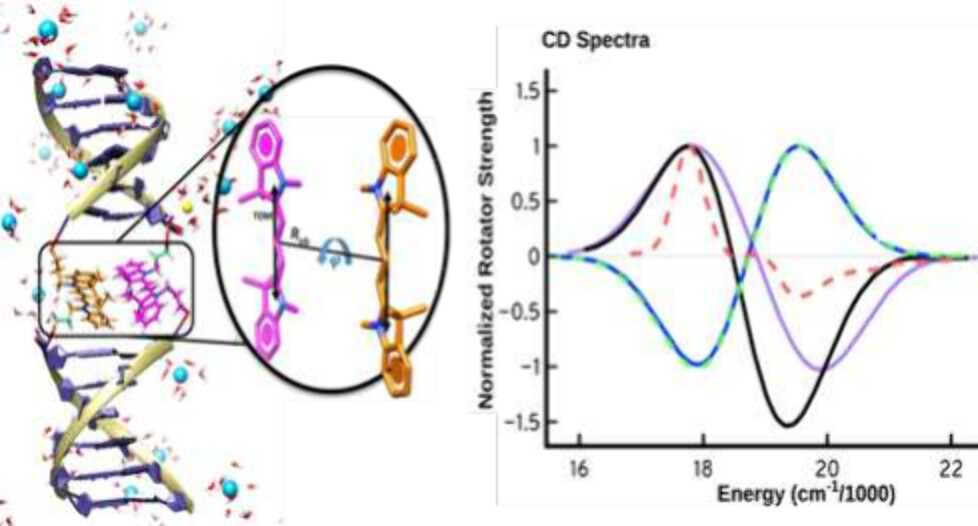
Molecular Dynamical and Quantum Mechanical Exploration of the Site-Specific Dynamics of Cy3 dimers internally linked to dsDNA
Mohammed I. Sorour, Kurt A. Kistler, Andrew H. Marcus, Spiridoula Matsika, J. Phys. Chem. B 2024, 128, 32, 7750–7760 (2024)
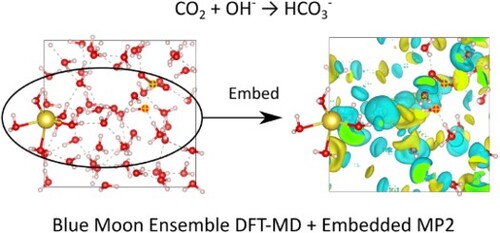
Modeling Bicarbonate Formation in an Alkaline Solution with Multi-Level Quantum Mechanics/Molecular Dynamics Simulations
B. Bobell, J.-N. Boyn, J. M. P. Martirez, and E. A. Carter, Molecular Physics Special Issue in Honour of Giovanni Ciccotti, Article: e2375370 (2024)
URL: doi.org/10.1080/00268976.2024.2375370
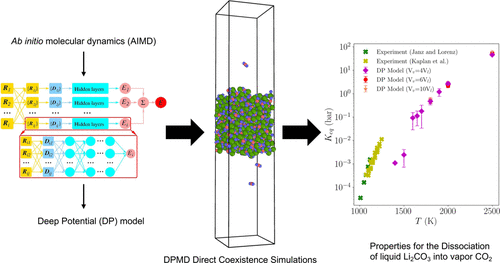
Molecular Simulation of Lithium Carbonate Reactive Vapor-Liquid Equilibria using a Deep Potential Model
D. Kussainova and A. Z. Panagiotopoulos, J. Chem. Eng. Data 69: 204-214 (2024)
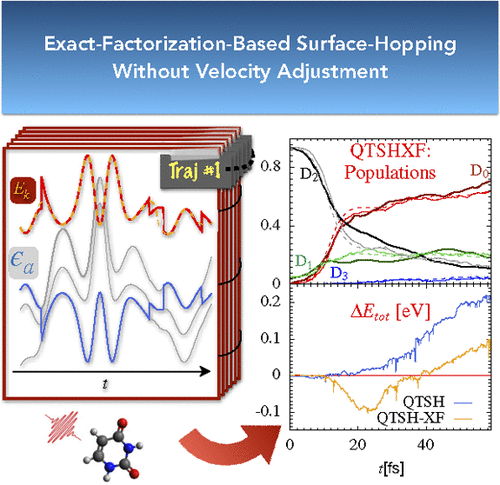
Exact-Factorization-Based Surface Hopping without Velocity Adjustment
Lucien Dupuy, Anton Rikus, Neepa T. Maitra, J. Phys. Chem. Lett. 15, 2643-2649 (2024)
Nonadiabatic dynamics with classical trajectories: The problem of an initial coherent superposition of electronic states
Evaristo Villaseco Arribas, Neepa T. Maitra, Federica Agostini, J. Chem. Phys. 160 (5) (2024)
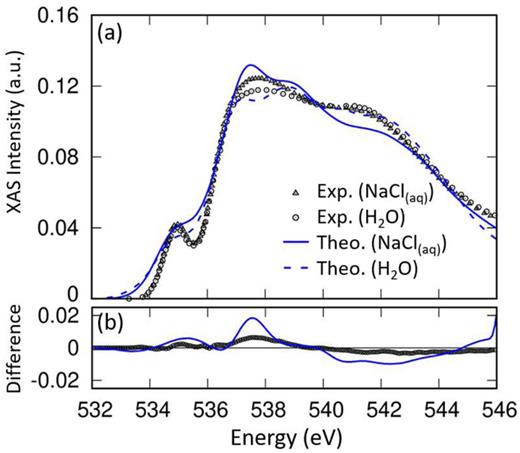
Exploring the impact of ions on oxygen K-edge X-ray absorption spectroscopy in NaCl solution using the GW-Bethe-Salpeter-equation approach
Fujie Tang, Kefeng Shi and Xifan Wu, The Journal of Chemical Physics, Volume 159, Issue 17 (2023)
URL: https://doi.org/10.1063/5.0167999
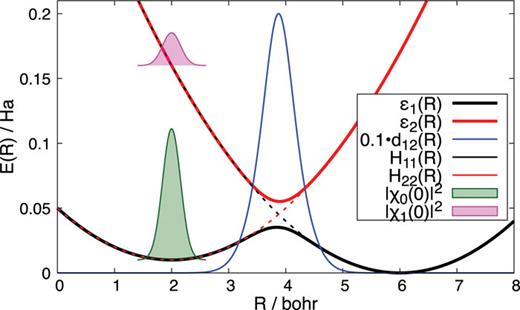
Ab Initio Generalized Langevin Equation
Pinchen Xie, Roberto Car and Weinan E, PNAS, 121 (14) e2308668121 (2024)

Probing the Self-ionization of Liquid Water with Ab-Initio Deep Potential Molecular Dynamics
Marcos Calegari Andrade, Roberto Car and Annabella Selloni, PNAS, 120 (46) e2302468120 (2023)

Elucidating the water–anatase TiO2(101) interface structure using infrared signatures and molecular dynamics
Christopher R. O’Connor, Marcos F. Calegari Andrade, Annabella Selloni and Greg A. Kimmel, J. Chem. Phys. 159, 104707 (2023)

Modeling the aqueous interface of amorphous TiO2 using deep potential molecular dynamics
Zhutian Ding and Annabella Selloni, J. Phys. Chem, 159, 024706 (2023)
URL: https://doi.org/10.1063/5.0157188

Acid–Base Chemistry of a Model IrO2 Catalytic Interface
Abhinav S. Raman and Annabella Selloni, J. Phys. Chem, Lett. 2023, 14, 35, 7787-7794 (2023)
URL: https://doi.org/10.1021/acs.jpclett.3c02001

Unravelling the Origin of the Vibronic Spectral Signatures in an Excitonically Coupled Indocarbocyanine Cy3 Dimer
Dimer Mohammed Sorour, Andrew Marcus, Spiridoula Matsika, J. Phys. Chem., 127, 45, 9530–9540A (2023)

Characterizing the Mechanisms of Ca and Mg Carbonate Ion-Pair Formation with Multi-Level Molecular Dynamics/Quantum Mechanics Simulations
Jan-Niklas Boyn and Emily A. Carter, J. Phys. Chem. B, 127, 50, 10824–10832 (2023)
URL: https://doi.org/10.1021/acs.jpcb.3c05369

Neural Network Water Model based on the MB-pol Many-Body Potential
Maria Muniz, Roberto Car, and Athanassios Panagiotopoulos, J. Phys. Chem. B, 127, 42, 9165-9171 (2023)

Probing pH-Dependent Dehydration Dynamics of Mg and Ca Cations in Aqueous Solutions with Multi-Level Quantum Mechanics/Molecular Dynamics Simulations
J.-N. Boyn and E. A. Carter, J. Am. Chem. Soc., 145, 37, 20462 (2023)

Significance of energy conservation in coupled-trajectory approaches to non-adiabatic dynamics
Evaristo Villaseco Arribas, Lea M. Ibele, David Lauvergnat, Neepa T. Maitra, Federica Agostini, J. Chem. Theory Comput. 2023, 19, 21, 7787–7800 (2023)
URL: https://chemrxiv.org/engage/chemrxiv/article-details/64ccb98f4a3f7d0c0d91f42a

Different Flavors of Exact-Factorization-Based Mixed Quantum-Classical Methods for Multistate Dynamics
Evaristo Villaseco Arribas, Patricia Vindel-Zandbergen, Saswata Roy, Neepa T. Maitra, accepted in Physical Chemistry Chemical Physics as “accepted manuscript” (2023)

Molecular Rotations, Multiscale Order, Hyperuniformity, and Signatures of Metastability during the Compression/Decompression Cycles of Amorphous Ices
Maud Formanek, Salvatore Torquato, Roberto Car, and Fausto Martelli, The Journal of Physical Chemistry B 2023, 127 (17), 3946-3957 (2023)

Critical behavior in a chiral molecular model
Pablo M. Piaggi, Roberto Car, Frank H. Stillinger, Pablo G. Debenedetti, J. Chem. Phys. 159, 114502 (2023)
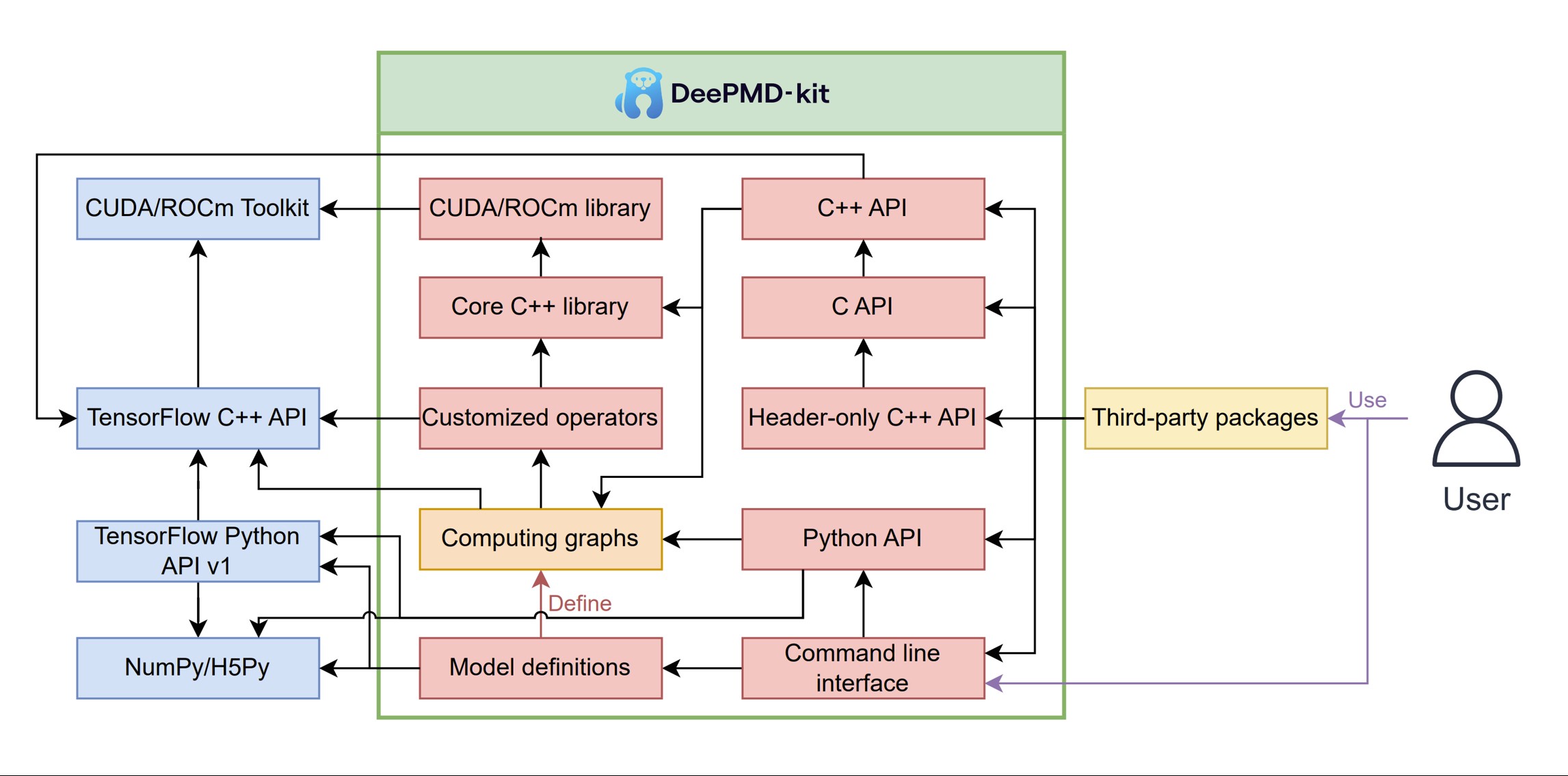
DeePMD-kit v2: A software package for Deep Potential models
Jinzhe Zeng, Duo Zhang, Denghui Lu, Pinghui Mo, Zeyu Li, Yixiao Chen, Marián Rynik, Li’ang Huang, Ziyao Li, Shaochen Shi, Yingze Wang, Haotian Ye, Ping Tuo, Jiabin Yang, Ye Ding, Yifan Li, Davide Tisi, Qiyu Zeng, Han Bao, Yu Xia, Jiameng Huang, Koki Muraoka, Yibo Wang, Junhan Chang, Fengbo Yuan, Sigbjørn Løland Bore, Chun Cai, Yinnian Lin, Bo Wang, Jiayan Xu, Jia-Xin Zhu, Chenxing Luo, Yuzhi Zhang, Rhys E. A. Goodall, Wenshuo Liang, Anurag Kumar Singh, Sikai Yao, Jingchao Zhang, Renata Wentzcovitch, Jiequn Han, Jie Liu, Weile Jia, Darrin M. York, Weinan E, Roberto Car, Linfeng Zhang, Han Wang, the Journal of Chemical Physics (Vol.159, Issue 5) (2023)

Thermal Conductivity of Water at Extreme Conditions
Cunzhi Zhang, Marcello Puligheddu, Linfeng Zhang, Roberto Car and Giulia Galli, J. Phys. Chem. B, 127, 31, 7011-7017 (2023)
URL: https://doi.org/10.1021/acs.jpcb.3c02972
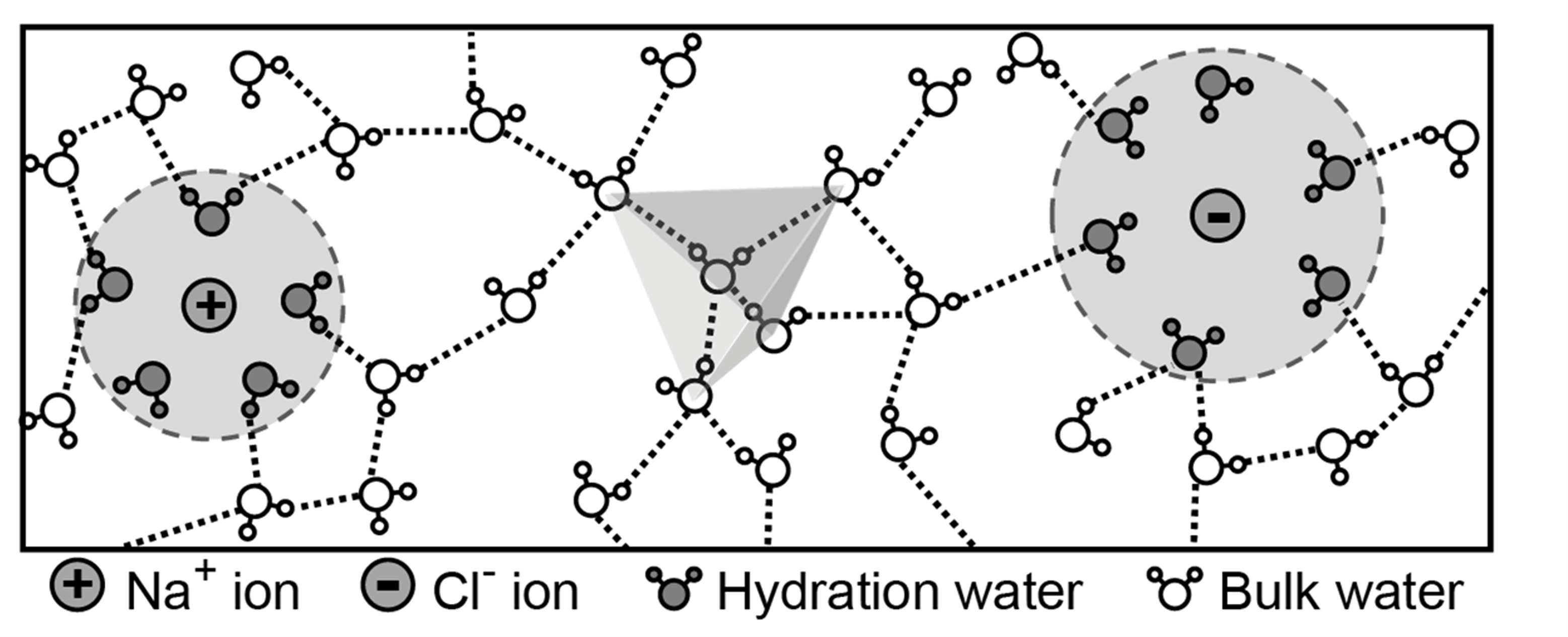
Why dissolving salt in water decreases its dielectric permittivity
Chunyi Zhang, Shuwen Yue, Athanassios Z. Panagiotopoulos, Michael L. Klein and Xifan Wu, Phys. Rev. Lett. 131, 076801 (2023)
URL: https://doi.org/10.1103/PhysRevLett.131.076801

A first-principles machine-learning force field for heterogeneous ice nucleation on microcline feldspar
Pablo M. Piaggi, Annabella Selloni, Athanassios Z. Panagiotopoulos, Roberto Car and Pablo G. Debenedetti, Faraday Discussions, 249: 98-113 (2024)
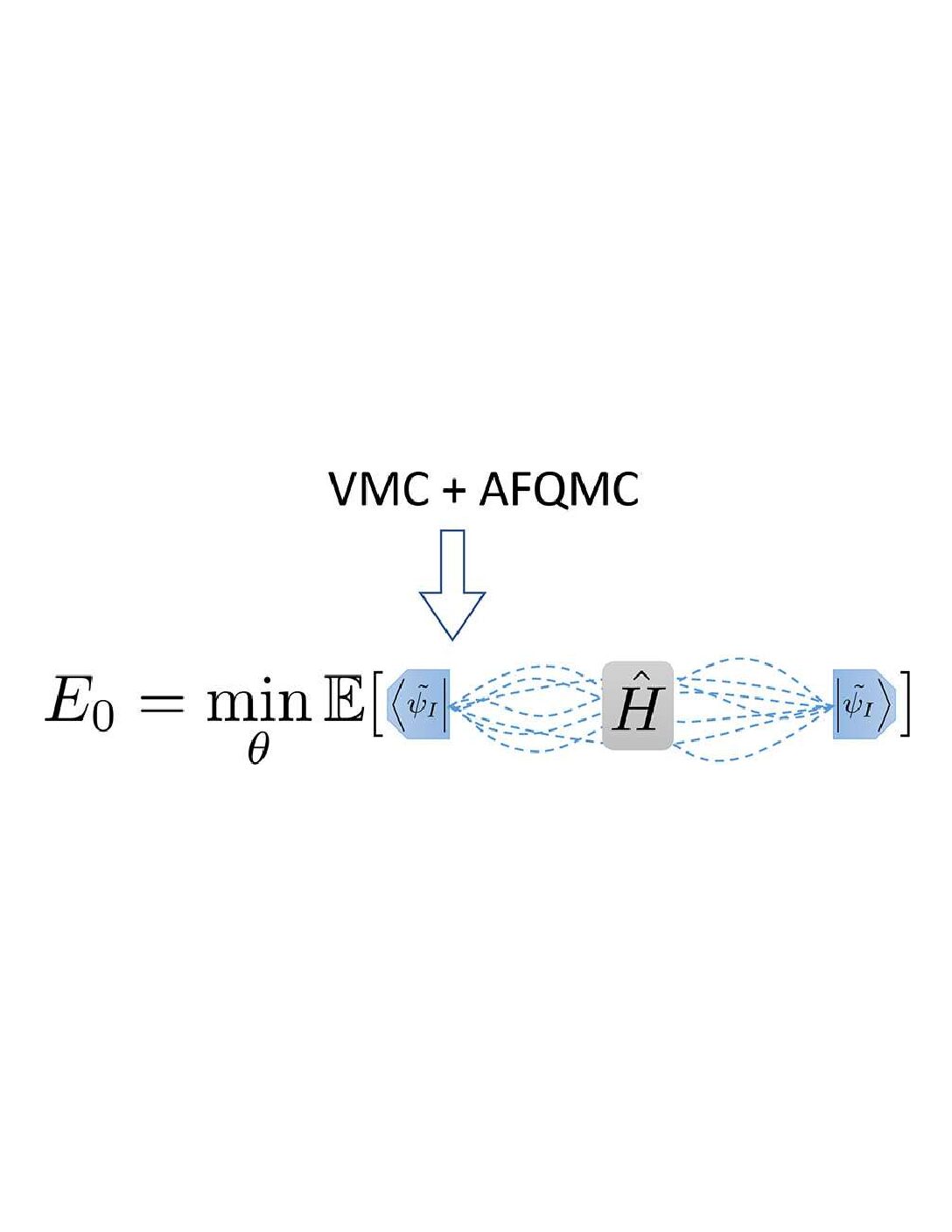
Hybrid Auxiliary Field Quantum Monte Carlo for Molecular Systems
Yixiao Chen, Linfeng Zhang, Weinan E. and Roberto Car, J. Chem. Theory Comput. 2 19, 14, 4484-4493 (2023)
URL: https://doi.org/10.1021/acs.jctc.3c00038

DeePKS+ ABACUS as a Bridge between Expensive Quantum Mechanical Models and Machine Learning Potentials
W. Li, Q. Ou, Y. Chen, Y. Cao, R. Liu, C. Zhang, D. Zheng, C. Cai, X. Wu, H. Wang, M. Chen, L. Zhang, The Journal of Physical Chemistry A, 126, 9154-9164 (2022)
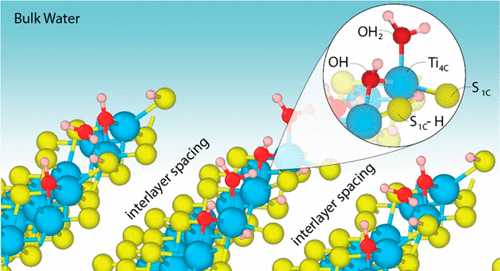
Characterizing Structure-dependent TiS2/Water Interfaces Using Deep-Neural-Network-Assisted Molecular Dynamics
L. Li, M.F. Calegari Andrade, R. Car, A. Selloni, E.A. Carter, J Phys Chem C 2023, 127, 9750-9758 (2023)

Dynamics of Aqueous Electrolyte Solutions: Challenges for Simulations
A. Z. Panagiotopoulos and S. Yue, J. Phys. Chem. B 127: 430-37 (2023)
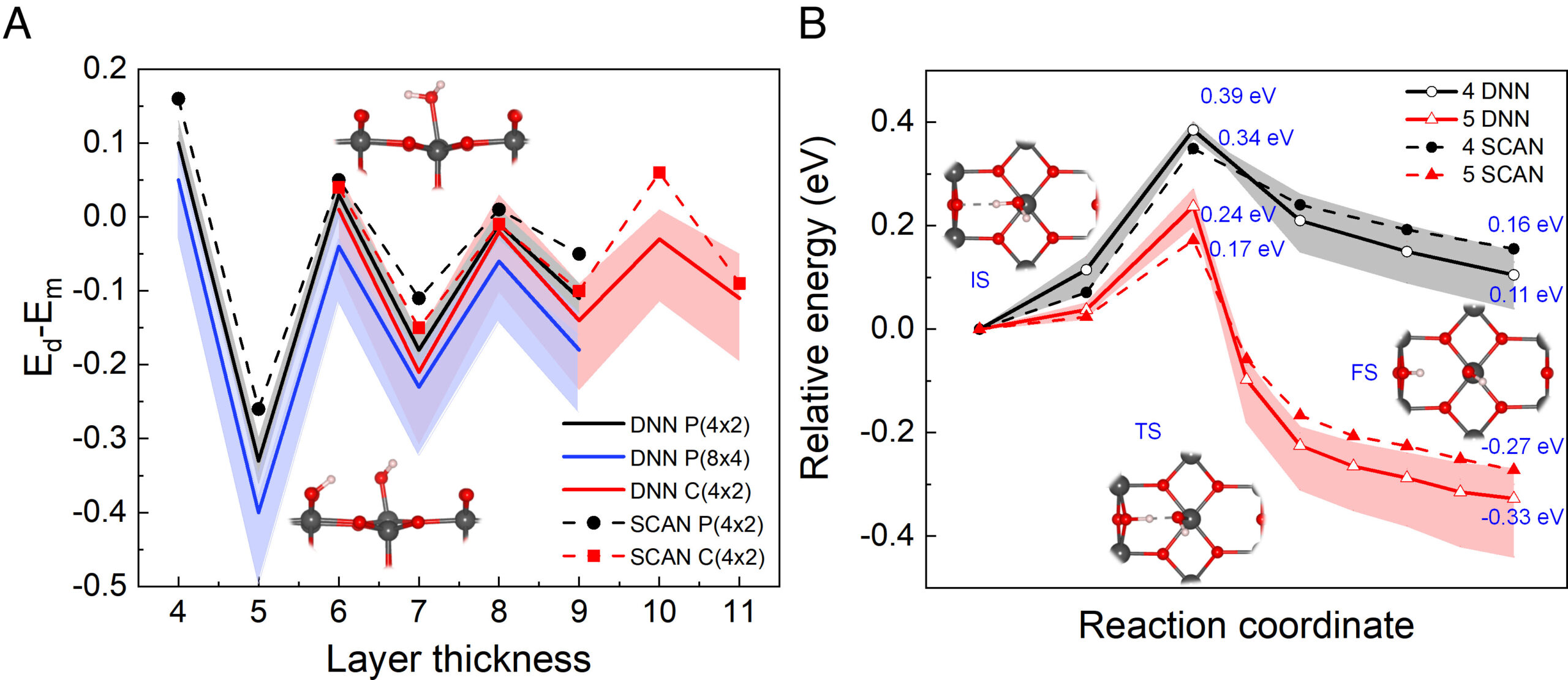
Water Dissociation at the Water-Rutile TiO2(110) Interface from Ab-initio based Deep Neural Network Simulations
B. Wen, M. F. Calegari Andrade, L.M. Liu, A. Selloni, Proc. Nat. Acad. Sci., 120, e2212250120 (2023)

Energy-Conserving Coupled-Trajectory Mixed-Quantum Classical Dynamics
Villaseco Arribas and N. T. Maitra, J. Chem. Phys. 158, 161105 (2023)

Characterization of Hole States at the Zn-doped Hematite/Water Interface from Ab Initio Simulations
Zachary K. Goldsmith, Zhutian Ding, and Annabella Selloni, ACS Catalysis 2023, 13, 8, 5298-5306 (2023)

Melting curves of ice polymorphs in the vicinity of the liquid-liquid critical point
Pablo M. Piaggi, Thomas E. Gartner III, Roberto Car, and Pablo G. Debenedetti, J. Chem. Phys. 159, 054502 (2023)

First-principles-based Machine Learning Models for Phase Behavior and Transport Properties of CO2
Reha Mathur, Maria Carolina Muniz, Shuwen Yue, Roberto Car and Athanassios Z. Panagiotopoulos, J. Phys. Chem. B, 2023, 127, 20, 4562–4569 (2023)
URL: https://doi.org/10.1021/acs.jpcb.3c00610

A Deep Potential model for liquid-vapor equilibrium and cavitation rates of water
Ignacio Sanchez-Burgos, Maria Carolina Muniz, Jorge R. Espinosa, and Athanassios Z. Panagiotopoulos, J. Chem. Phys. 158, 184504 (2023)

Liquid-liquid transition in water from first principles
Thomas E. Gartner III, Pablo M. Piaggi, Roberto Car, Athanassios Z. Panagiotopoulos and Pablo G. Debenedetti, Phys. Rev. Lett. 129, 255702 (2022)
URL: https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.129.255702

Modeling the Electronic Absorption Spectra of the Indocarbocyanine Cy3
Mohammed Sorour, Andrew Marcus and Spiridoula Matsika, Molecules 2022, 27(13), 4062 (2022)

Oxygen-Chlorine Chemisorption Scaling for Seawater Electrolysis on Transition Metals: The Role of Redox
Robert B. Wexler and Emily A. Carter, Advanced Theory and Simulations/Early Review/Research Article, 2200592 (2022)
URL: https://doi.org/10.1002/adts.202200592

Modeling the Solvation and Acidity of Carboxylic Acids Using an Ab Initio Deep Neural Network Potential
Abhinav S. Raman and Annabella Selloni, J. Phys. Chem. A 2022, 126, 40, 7283–7290 (2022)

A Tensor Network Path Integral Study of Dynamics in B850 LH2 Ring with Atomistically Derived Vibrations
Amartya Bose and Peter L. Walters, J. Chem. Theory Comput. 2022, 18, 7, 4095–4108 (2022)

Effect of Temperature Gradient on Quantum Transport
Amartya Bose and Peter L. Waters, Phys. Chem. Chem. Phys., 2022,24, 22431-22436 (2022)
URL: https://doi.org/10.1039/D2CP03030F

Phase diagram of the TIP4P/Ice water model by enhanced sampling simulations
Sigbjørn L Bore, Pablo M Piaggi, Roberto Car, Francesco Paesani, J. Chem. Phys. 157, 054504 (2022)
URL: https://doi.org/10.1063/5.0097463

Exact Factorization Adventures: A Promising Approach for Non-Bound State
Evaristo Villaseco Arribas, Federica Agostini, and Neepa T. Maitra, Molecules (for a special issue on Molecular Quantum Dynamics Beyond Bound States), Molecules 2022, 27(13), 4002 (2022)

Many-Body Effects in the X-ray Absorption Spectra of Liquid Water
Fujie Tang, Zhenglu Li, Chunyi Zhang, Steven G. Louie, Roberto Car, Diana Y. Qiu and Xifan Wu, Proc. Natl. Acad. Sci. U.S.A., 2022, 119, e2201258119 (2022)
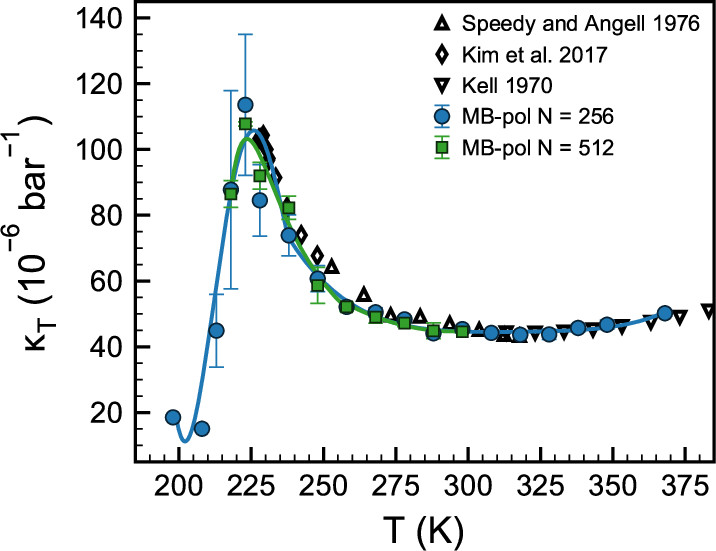
Anomalies and Local Structure of Liquid Water from Boiling to the Supercooled Regime as Predicted by the Many-Body MB-pol Model
Thomas E. Gartner III, Kelly M. Hunter, Eleftherios Lambros, Alessandro Caruso, Marc Riera, Gregory R. Medders, Athanassios Z. Panagiotopoulos, Pablo G. Debenedetti and Francesco Paesani, J. Phys. Chem. Lett. 2022, 13, XXX, 3652–3658 (2022)
URL: https://pubs.acs.org/doi/full/10.1021/acs.jpclett.2c00567
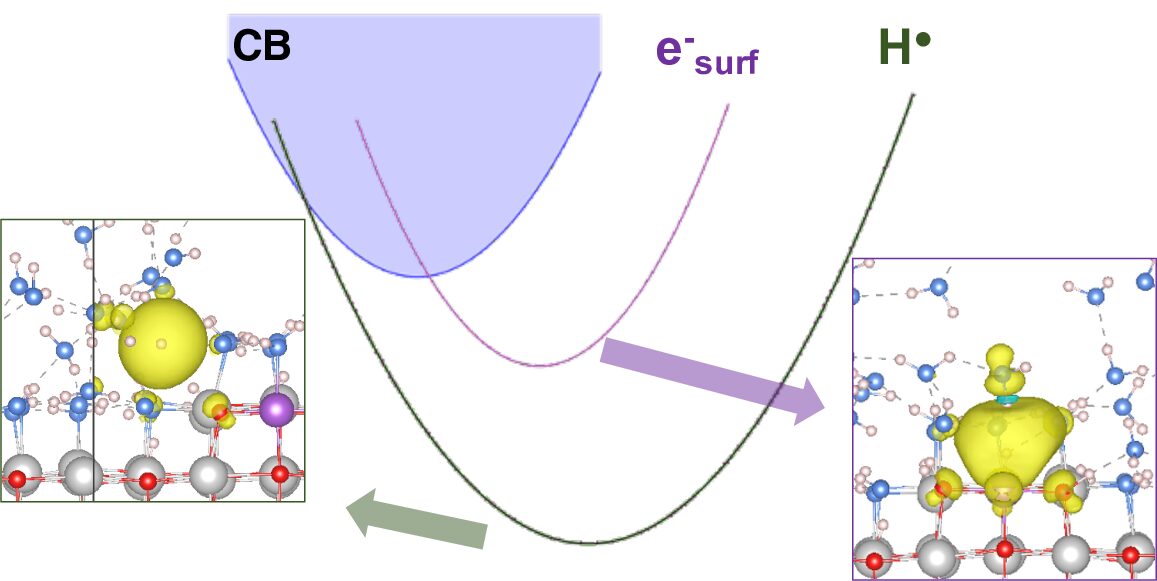
Pathways for Electron Transfer at MgO-Water Interfaces from Ab-Initio Molecular Dynamics
Zhutian Ding, Zachary K Goldsmith and Annabella Selloni, J. Am. Chem. Soc. 144, 2002-2009 (2022)
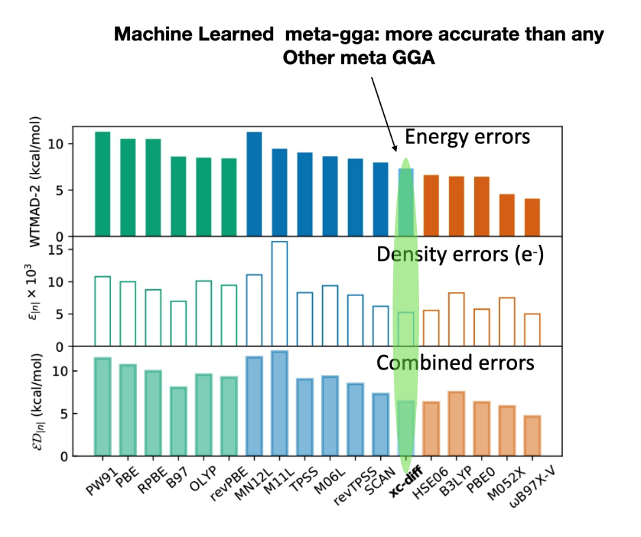
Highly accurate and constrained density functional obtained with differentiable programming
Sebastian Dick and Marivi Fernandez-Serra, Phys Rev B 104, L161109 (2021)
![A deep potential model with long-range electrostatic interactions [Editor’s pick]](https://ccsc.princeton.edu/wp-content/uploads/2022/01/5.0083669.figures.online.f5.jpeg)
A deep potential model with long-range electrostatic interactions [Editor’s pick]
L Zhang, H Wang, MC Muniz, AZ Panagiotopoulos, R. Car, W. E, J. Chem. Phys. 156, 124107 (2022)
URL: https://doi.org/10.1063/5.0083669
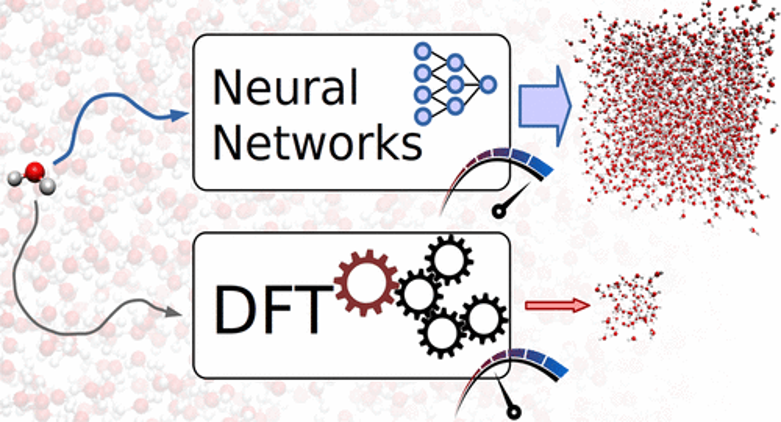
Using Neural Networks force fields to ascertain the quality of ab initio simulations of liquid water
Alberto Torres, Luana S. Pedroza, Marivi Fernandez-Serra,and Alexandre R.Rocha, submitted to J. Phys. Chem. C (2021)

Homogeneous ice nucleation in an ab-initio machine learning model of water
Pablo M. Piaggi, Jack Weiss, Athanassios Z. Panagiotopoulos, Pablo G. Debenedetti, Roberto Car, PNAS, 119 (33) e2207294119 (2022)
URL: https://doi.org/10.1073/pnas.2207294119

Viscosity in water from first-principles and deep-neural-network simulations
Cesare Malosso, Linfeng Zhang, Roberto Car, Stefano Baroni, Davide Tisi, npj Computational Materials, volume 8, Article number: 139 (2022)
URL: https://www.nature.com/articles/s41524-022-00830-7
Dissolving salt is not equivalent to applying a pressure on water
Chunyi Zhang, Shuwen Yue, Athanassios Z. Panagiotopoulos, Michael L. Klein, and Xifan Wu, Nature Communications 13, Article number: 822 (2022)

A pairwise connected tensor network representation of path integrals
Amartya Bose, Phys. Rev. B 105, 024309 (2022)

A Computational Study of RNA Tetraloop Thermodynamics, Including Misfolded States
Gül H. Zerze, Pablo M. Piaggi, and Pablo G. Debenedetti
J. Phys. Chem. B 125, 50, 13685-13695 (2021)

Heat transport in liquid water from first-principles and deep neural network simulations
Davide Tisi, Linfeng Zhang, Riccardo Bertossa, Han Wang, Roberto Car, and Stefano Baroni, Phys. Rev. B 104, 224202 – Published 13 (2021)
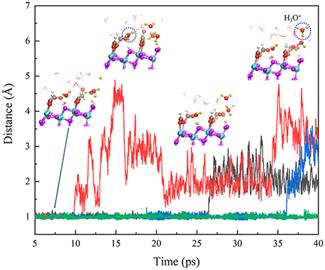
Hydrogen Bonds and H3O+ Formation at the Water Interface with Formic Acid Covered Anatase TiO2
Bo Wen, and Annabella Selloni, J Phys Chem Lett., 12, 6840-6846 (2021)
URL:https://pubs.acs.org/doi/abs/10.1021/acs.jpclett.1c01886
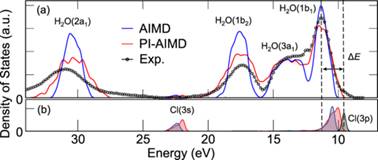
Nuclear quantum effects on the quasiparticle properties of the chloride anion aqueous solution within the GW approximation
Fujie Tang, Jianhang Xu, Diana Y. Qiu, and Xifan Wu,, Phys. Rev. B 104, 035117 (2021)

Exact-Factorization-Based Surface-Hopping for Multi-State Dynamics
Patricia Vindel-Zandbergen, Spiridoula Matsika and Neepa T. Maitra, . Phys. Chem. Lett. 2022, 13, 7, 1785–1790 (2022)

A Multisite Decomposition of the Tensor Network Path Integrals
Amartya Bose and Peter L. Walters, accepted by J. Chem. Phys. 156, 024101 (2022)

Modeling the Ultrafast Electron Attachment Dynamics of Solvated Uracil
Cate S. Anstöter, Mark DelloStritto, Michael L. Klein, and Spiridoula Matsika, J. Phys. Chem. A 2021, 125, 32, 6995–7003 (2021)

Modeling liquid water by climbing up Jacob’s ladder in density functional theory facilitated by using deep neural network potentials
Chunyi Zhang, Fujie Tang, Mohan Chen, Jianhang Xu, Linfeng Zhang, Diana Y. Qiu, John P. Perdew, Michael L. Klein, and Xifan Wu, J. Phys. Chem. B 2021, 125, 11444−11456 (2021)
URL:https://https://doi.org/10.1021/acs.jpcb.1c03884

Hydration structure of flat and stepped MgO surfaces
Zhutian Ding, and Annabella Selloni, J. Chem. Phys. 154, 114708 (2021)

Phase Diagram of a Deep Potential Water Model
Linfeng Zhang, Han Wang, Roberto Car, and Weinan E, Phys. Rev. Lett. 126, 236001 (2021)
URL:https://link.aps.org/doi/10.1103/PhysRevLett.126.236001

Vapor–liquid equilibrium of water with the MB-pol many-body potential
Maria Carolina Muniz, Thomas E. Gartner III, Marc Riera, Christopher Knight, Shuwen Yue, Francesco Paesani, and Athanassios Z. Panagiotopoulos, J. Chem. Phys. 154, 211103 (2021)
URL:https://aip.scitation.org/doi/10.1063/5.0050068?via=site

A study of the decoherence correction derived from the exact factorization approach for non-adiabatic dynamics
Patricia Vindel-Zandbergen, Lea M. Ibele, Jong-Kwon Ha, Seung Kyu Min, Basile F.E. Curchod, Neepa T. Maitra, J. Chem. Theory Comput. 2021, 17, 3852−3862 (2021)

Manifestations of metastable criticality in the long-range structure of model water glasses
Thomas E. Gartner III, Salvatore Torquato, Roberto Car, and Pablo G. Debenedetti, Nature Communications, 12, 3398 (2021)

Effects of applied voltage on water at a gold electrode interface from ab initio molecular dynamics
Zachary K. Goldsmith, Marcos F. Calegari Andrade, Annabella Selloni, Chem. Sci. (2021)
URL: https://pubs.rsc.org/en/content/articlelanding/2021/SC/D1SC00354B#!divAbstract
Phase equilibrium of water with hexagonal and cubic ice using the SCAN functional
PM Piaggi, AZ Panagiotopoulos, PG Debenedetti, and R Car, J. Chem. Theory Comput. 17, 5, 3065–3077 (2021)

Enhancing the formation of ionic defects to study the ice Ih/XI transition with molecular dynamics simulations
PM Piaggi and R Car, Mol. Phys. e1916634 (2021)

Quantum phase transitions in long-range interacting hyperuniform spin chains in a transverse field
Amartya Bose and Salvatore Torquato, Phys. Rev. B 103, 014118 (2021)
URL: https://journals.aps.org/prb/abstract/10.1103/PhysRevB.103.014118

When do short-range atomistic machine-learning models fall short?
Shuwen Yue*, Maria Carolina Muniz*, Marcos F. Calegari Andrade, Linfeng Zhang, Roberto Car, and Athanassios Z. Panagiotopoulos, J. Chem. Phys. 154, 034111 (2021)
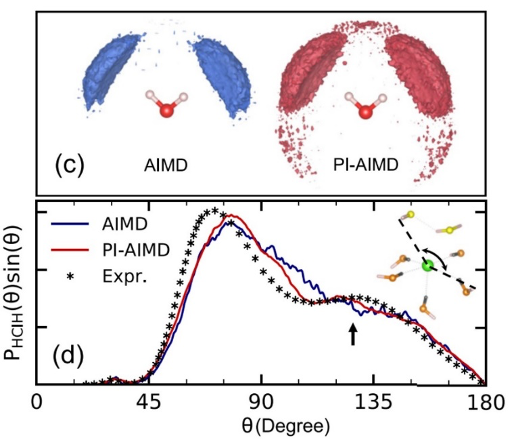
Importance of nuclear quantum effects on the hydration of chloride ion
Jianhang Xu, Zhaoru Sun, Chunyi Zhang, Mark DelloStritto, Deyu Lu, Michael L. Klein, and Xifan Wu, Physical Review Materials, 5, L012801 (2021)
URL: https://journals.aps.org/prmaterials/abstract/10.1103/PhysRevMaterials.5.L012801
DeePKS: A Comprehensive Data-Driven Approach toward Chemically Accurate Density Functional Theory
Yixiao Chen, Linfeng Zhang, Han Wang, and Weinan E, Journal of Chemical Theory and Computation, 2021, 17, 1, 170–181 (2020)

Electron Trapping and Ion Leaching at the Li-Modified Quartz–Water Interface*
Zhutian Ding and Annabella Selloni, J. Phys. Chem. C 2020, 124, 49, 26741–26747 (2020)
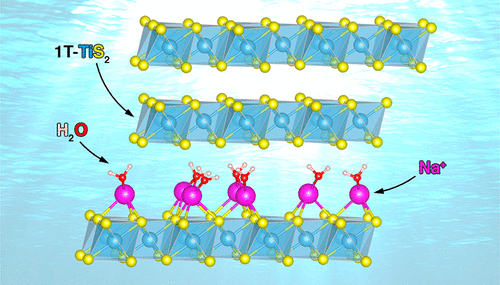
First-Principles Modeling of Sodium Ion and Water Intercalation into Titanium Disulfide Interlayers for Water Desalination
Lesheng Li, Shenzhen Xu and Emily A. Carter, Chem. Mater. 2020, 32, 24, 10678–10687 (2020)

Isotope effects in molecular structures and electronic properties of liquid water via deep potential molecular dynamics based on the SCAN functional
Jianhang Xu, Chunyi Zhang, Linfeng Zhang, Mohan Chen, Biswajit Santra, and Xifan Wu, Physical Review B 102, 24113 (2020)

Signatures of a liquid-liquid transition in an ab-initio neural network model for water
Thomas E. Gartner III, Linfeng Zhang, Pablo M. Piaggi, Roberto Car, Athanassios Z. Panagiotopoulos, and Pablo G. Debenedetti , PNAS, 117 (42) 26040-26046 (2020)

Hydrogen dynamics in supercritical water probed by neutron scattering and computer simulations
Carla Andreani, Giovanni Romanelli, Alexandra Parmentier, Roberto Senesi, Alexander I. Kolesnikov, Hsin-Yu Ko, Marcos Calegari Andrade, and Roberto Car, Hydrogen dynamics in supercritical water probed by neutron scattering and computer simulations, J. Phys. Chem. Lett. 11, 9461–9467 (2020)
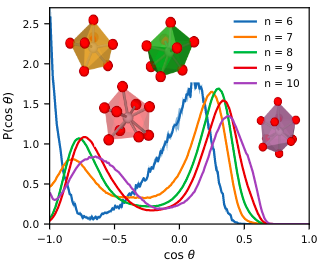
Structure of disordered TiO2 phases from ab initio based deep neural network simulations
Marcos Calegari Andrade, M. F. & Selloni, A. Structure of disordered TiO2 phases from ab initio based deep neural network simulations, Phys. Rev. Mater. 4, 113803 (2020)
URL: https://doi.org/10.1103/PhysRevMaterials.4.113803

Proton-transfer dynamics in ionized water chains using real-time Time Dependent Density Functional Theory
Vidushi Sharma and Marivi Fernández-Serra, Phys. Rev. Research 2, 043082 (2020)

Machine Learning a Highly Accurate Exchange and Correlation Functional of the Electronic Density
Sebastian Dick and Marivi Fernandez-Serra, Nat Commun 11, 3509 (2020)
Understanding the Interplay Between the Non-Valence and Valence State of the Uracil Anion Upon Mono-Hydration
Cate S. Anstöter and Spiridoula Matsika, J. Phys. Chem. A, 2020, 124, 44, 9237-9243 (2020)

Ground state energy functional with Hartree-Fock efficiency and chemical accuracy
Yixiao Chen, Linfeng Zhang, Han Wang, and Weinan E, J. Phys. Chem. A 2020, 124, 35, 7155–7165 (2020)

Isotope effects in x-ray absorption spectra of liquid water
Chunyi Zhang, Linfeng Zhang, Jianhang Xu, Fujie Tang, Biswajit Santra, and Xifan Wu, Physical Review B 102, 115155 (2020)

Hydration of NH+4 in water: bifurcated hydrogen bonding structures and fast rotational dynamics
Jianqing Guo, Liying Zhou, Andrea Zen, Angelos Michaelides, Xifan Wu, Enge Wang, Limei Xu, and Ji Chen, Phys. Rev. Lett. 125, 106001, (2020)

Stabilization of Hydroxide Ion at Interface of Hydrophobic Monolayer on Water via Reduced Proton Transfer
Shanshan Yang, Mohan Chen, Yudan, Su, Jianhang Xu, Xifan Wu, and Chuanshan Tian, Phys. Rev. Lett. 125, 156803, (2020)
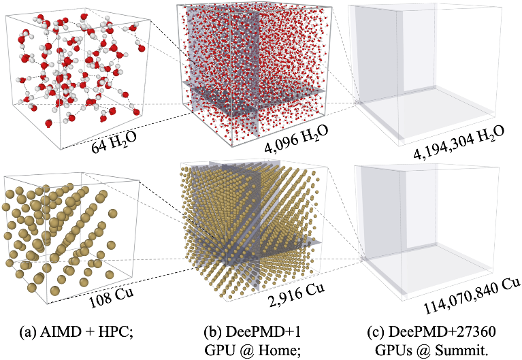
86 PFLOPS Deep Potential Molecular Dynamics simulation of 100 million atoms with ab initio accuracy
D Lu, H Wang, M Chen, J Liu, L Lin, R Car, W. E, W Jia, L Zhang
Comp. Phys. Comm., vol 259, February 2021, 107624. (2021)

Isotope effects in liquid water via deep potential molecular dynamics
Hsin-Yu Ko, Linfeng Zhang, Biswajit Santra, Han Wang, Weinan E, Robert A DiStasio Jr, Roberto Car
Molecular Physics, 11(22), 3269-3281 (2019)

Neural Canonical Transformation with Symplectic Flows
Shuo-Hui Li, Chen-Xiao Dong, Linfeng Zhang, and Lei Wang, Physical Review X 10, 021020 (2020)
URL: DOI: 10.1103/PhysRevX.10.021020

DP-GEN: A concurrent learning platform for the generation of reliable deep learning based potential energy models
Y. Zhang, H. Wang, W. Chen, J. Zeng, L. Zhang, H. Wang, W. E
Comp. Phys. Comm. (2020)
URL: https://www.sciencedirect.com/science/article/pii/S001046552030045X

Aqueous Solvation of the Chloride Ion Revisited with Density Functional Theory: Impact of Correlation and Exchange Approximations
Mark DelloStritto, Jianhang Xu, Xifan Wu and Michael L. Klein, Phys. Chem. Chem. Phys., 2020, 22, 10666-10675 (2020)

Phase equilibrium of liquid water and hexagonal ice from enhanced sampling molecular dynamics simulations
Pablo M. Piaggi and Roberto Car, J. Chem. Phys. 152, 204116 (2020)

Raman spectrum and polarizability of liquid water from deep neural networks
Grace M. Sommers, Marcos F. Calegari Andrade, Linfeng Zhang, Han Wang and Roberto Car, R.
Phys. Chem. Chem. Phys. 2020, 22, 10592-10602 (2020)
URL: DOI: 10.1039/D0CP01893G

Enabling Large-Scale Condensed Phase Hybrid Density Functional Theory Based Ab Initio Molecular Dynamics. I. Theory, Algorithm, and Performance
Hsin-Yu Ko, Junkeng Jia, Biswajit Santra, Xifan Wu, Roberto Car, and Robert A. DiStasio Jr., J. Chem. Theory Comput. 2020, 16, 6, 3757–3785 (2020)

Free energy of proton transfer at the water–TiO2 interface from ab initio deep potential molecular dynamics
Marcos F. Calegari Andrade, Hsin-Yu Ko, Linfeng Zhan, Roberto Car, Annabella Selloni, Chemical Science, 11(9), 2335–2341 (2020)

Deep neural network for the dielectric response of insulators
Linfeng Zhang, Mohan Chen, Xifan Wu, Han Wang and Roberto Car, Phys. Rev. B, 102, 04112 (R) (2020)

Learning from the Density to Correct Total Energy and Forces in First Principle Simulations
Sebastian Dick and Marivi Fernandez-Serra, J. Chem. Phys. 151, 144102 (2019)
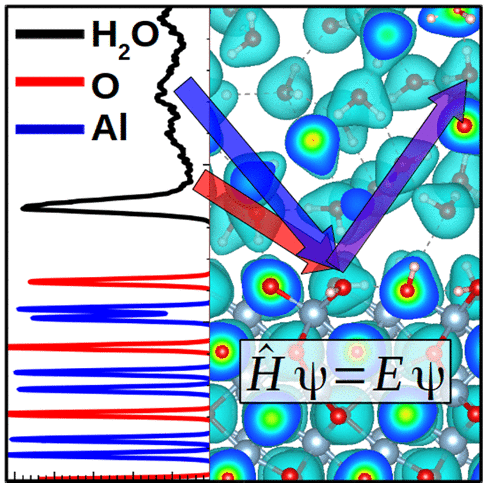
Effect of Functional and Electron Correlation on the Structure and Spectroscopy of the Al2O3(001)-H2O Interface
Mark J. DelloStritto, Stephan M. Piontek, Michael L. Klein, and Eric Borguet, J. Phys. Chem. Lett., 2019, 10, 9, 2031–2036 (2019)

Active learning of uniformly accurate interatomic potentials for materials simulation
Linfeng Zhang, De-Ye Lin, Han Wang, Roberto Car, Weinan E., Phys. Rev. Mat. 3, 023804 (2019)
URL: https://doi.org/10.1103/PhysRevMaterials.3.023804

Importance of van der Waals effects on the hydration of metal ions from the Hofmeister series
Liying Zhou, Jianhang Xu, Limei Xu and Xifan Wu, J. Chem. Phys. 150, 124505 (2019)

Structure, Polarization, and Sum Frequency Generation Spectrum of Interfacial Water on Anatase TiO2
Marcos F. Calegari Andrade, Hsin-Yu Ko, Roberto Car, Annabella Selloni, J. Chem. Phys. Lett. 9, 23, 6716 – 6721 (2018)
